CIDRZ Joins the World in Commemorating World TB Day.
March 24, 2025
CIDRZ Joins World Health Day Commemoration in Lusaka
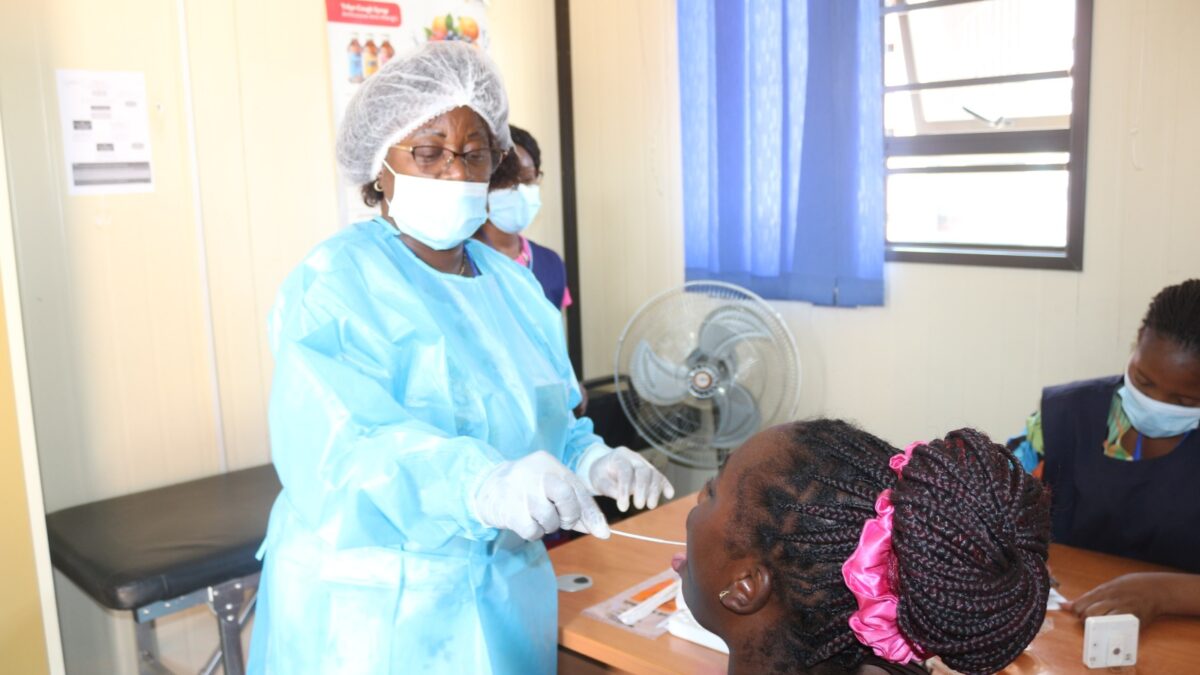
April 7, 2025The Centre for Infectious Diseases Research in Zambia (CIDRZ) Chawama Clinical Research Site (CRS) has successfully identified, diagnosed, and referred over 100 patients for tuberculosis (TB) treatment through the Assessing Diagnostics at Point-of-Care for Tuberculosis (ADAPT) study.
This initiative supports national and global efforts to improve TB detection and care in high-burden countries.
The ADAPT study seeks to evaluate new point-of-care (POC) TB diagnostic tests in these high-burden countries to enhance the speed of diagnosis, treatment, and patient outcomes.
The study assesses novel TB diagnostic platforms, including Truenat MTB, Rapi-Q, and Standard M10. Findings from the Adapt Study will guide further evaluation and potential deployment of these technologies following review by National TB Programs (NTPs) and the World Health Organization (WHO).
CIDRZ Chief Scientific Officer Dr Monde Muyoyeta stated that the results of the study will be shared both locally and internationally through conference presentations and publications.
“The results of this study will be disseminated locally and internationally through conference presentations and publications. Additionally, data will be shared with WHO for review and consideration for pre-qualification of new point-of-care tests,” she said.