CIDRZ and Teledoctor Zambia Sign MoU to Advance Healthcare Access and Innovation.
June 10, 2025
CIDRZ, MoH Strengthen PMTCT Efforts Through Intensive Cohort Monitoring Training in Lusaka
June 12, 2025The Centre for Infectious Disease Research in Zambia (CIDRZ) is leading a landmark study that is transforming the understanding and clinical management of liver disease in people co-infected with HIV and Hepatitis B Virus (HBV) in Zambia.
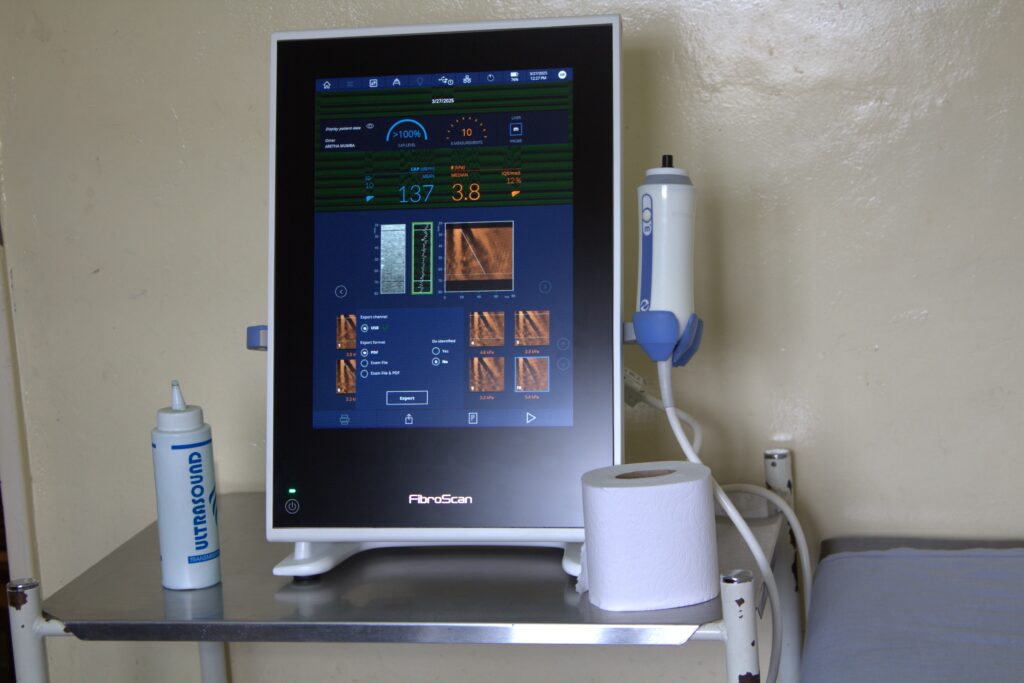
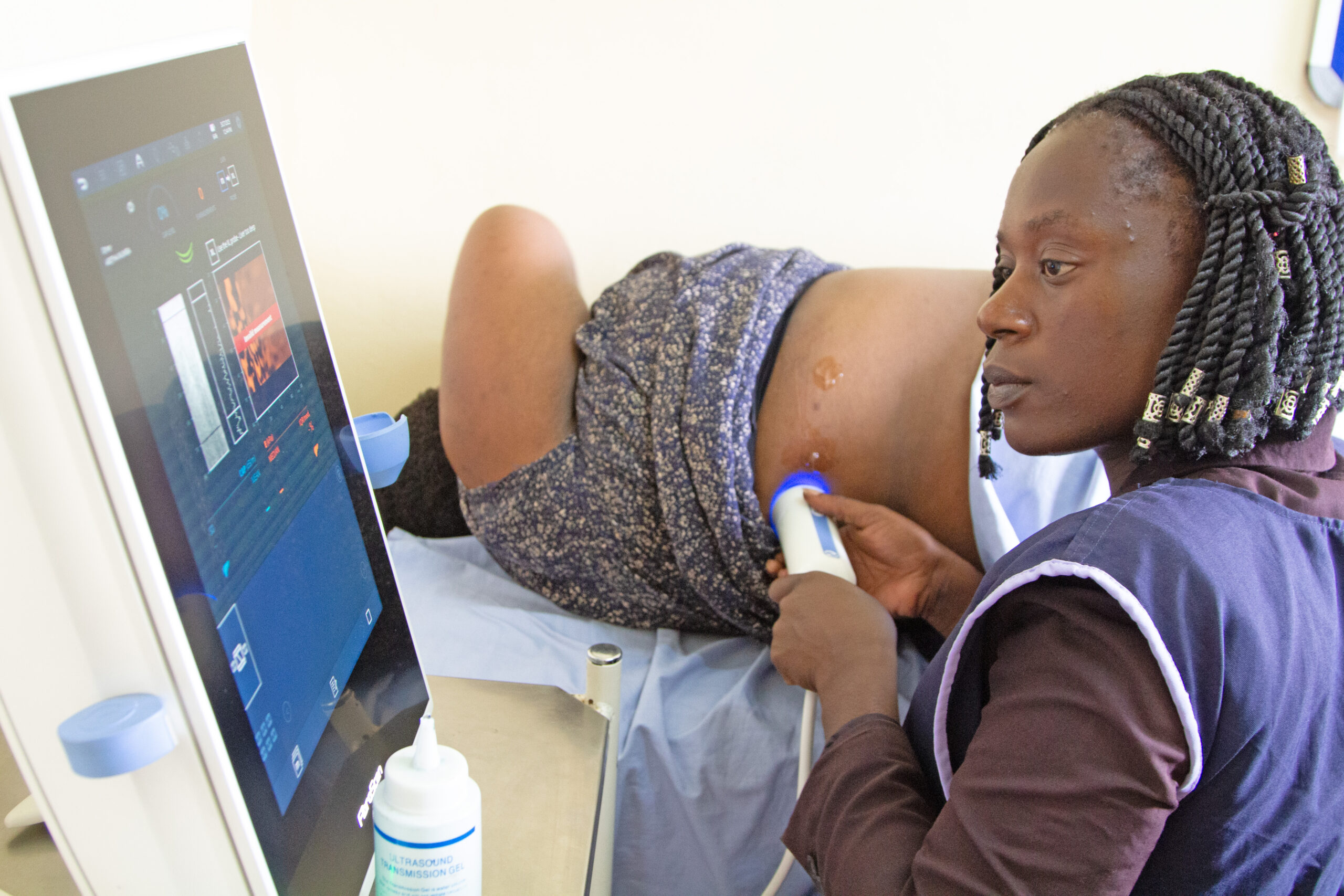
With HIV-HBV co-infection affecting up to 2% of Zambian adults, CIDRZ’s ongoing study, launched in 2015 and funded by National Institutes of Health (NIH) and the International Epidemiology Databases to Evaluate AIDS (IeDEA-SA), is the first in Zambia to assess the prevalence and predictors of liver fibrosis using non-invasive tests, and the impact of ART on disease progression.
Dr Guy Muula, CIDRZ IeDEA Research Physician, said this 10-year grant-funded initiative aims at closing critical knowledge gaps on HIV and Hepatitis B Virus (HBV) co-infection in Zambia.
“This study was initially launched at Matero First Level Hospital and later transitioned to Kanyama First Level Hospital. The project assesses the impact of antiretroviral therapy (ART) on liver fibrosis progression and examines the rate and predictors of Hepatitis B surface antigen (HBsAg) clearance in ART-treated patients,” he explained.
Dr Muula added that over the years, the programme has been systematically executed, ensuring robust data collection and effective patient care.
He noted that these findings will enhance understanding and guide improved management strategies for individuals co-infected with HIV and HBV.
“Key findings so far show that half of co-infected patients begin treatment with high HBV viral loads, placing them at increased risk of liver cancer. Encouragingly, over 85% of patients on ART achieve viral suppression, and HBsAg clearance rates are higher than expected, indicating strong immune recovery,” he said.
The study has so far influenced national Hepatitis B guidelines, leading to the creation of specialised HBV clinics, and enhanced clinician training. As it enters its final year, the research continues to shape policy and improve care for co-infected patients in Zambia and beyond.